Anode and Cathode in Electrolysis
Published continuously from 1902 to the present JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology. The cathode is the current that leaves the electrodes or cathode is a result of reduction reaction taking place in an electrolyte mixture.

Chemistry Subject Nickel Electroplating System Electronic Circuit Projects Chemistry Electroplating
It is the positive electrode meaning the electrons flow from the electrical circuit through the cathode into the non-metallic part of the electrochemical cell.

. A cathode is a negatively charged electrode. The dotted vertical line in the above figure represents a diaphragm that prevents the Cl 2 produced at the anode in this cell from coming into contact with the NaOH that accumulates at the cathode. Electrowinning is the oldest industrial electrolytic process.
The electrolysis takes place at a much lower potential than pure water 24V. The anode is a positively charged electrode. Electrolysis involves the manipulation of chemical reactions based on their electric potential.
Electroplating and electrolysis welding cathodic protection. The reaction between the two elements in an electrolytic cell is a reduction-oxidation -- or redox -- reaction. The cathode is in many ways the opposite of the anode.
Before we learn about the terms cathode and anode it is important to understand what an electrode is. The more noble metal in the reaction will always be the cathode. The anode is the site where.
The process of electrolysis sees electrons being stripped from the anode. Then they head towards the cathode. The two common terms we hear is cathode and anode.
Cathode Anode and Electrolyte. Half reactions of electrolysis in the presence of a base are-. The English chemist Humphry Davy obtained sodium metal in elemental form for the first time in 1807 by the electrolysis of molten sodium hydroxide.
When this diaphragm is removed from the cell the products of the electrolysis of aqueous sodium chloride react to form sodium hypo-chlorite which is the first step in the. Water Electrolysis in the Presence of a Base pH higher than 7 Additional hydroxyl ions release their electrons to anode while electrons at the cathode oxidize water molecules near it. In electrochemical cells the cathode is the site where reduction occurs.
James Elkington patented the commercial process. The name also coined by William Whewell comes from the Greek words κάθά kata downwards and ὁδός hodós a way. According to the general definition an electrode is a substance that helps in electricity conduction wherein the electric current either leaves or enters the non-metallic medium such as an electrolytic cell.
Here electrons are released from the electrode and the surrounding solution is reduced. Electrorefining of copper was first demonstrated experimentally by Maximilian Duke of Leuchtenberg in 1847. JES is the flagship journal of The Electrochemical Society.
So what does that mean. The less noble metal in a reaction will be the anode.

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions
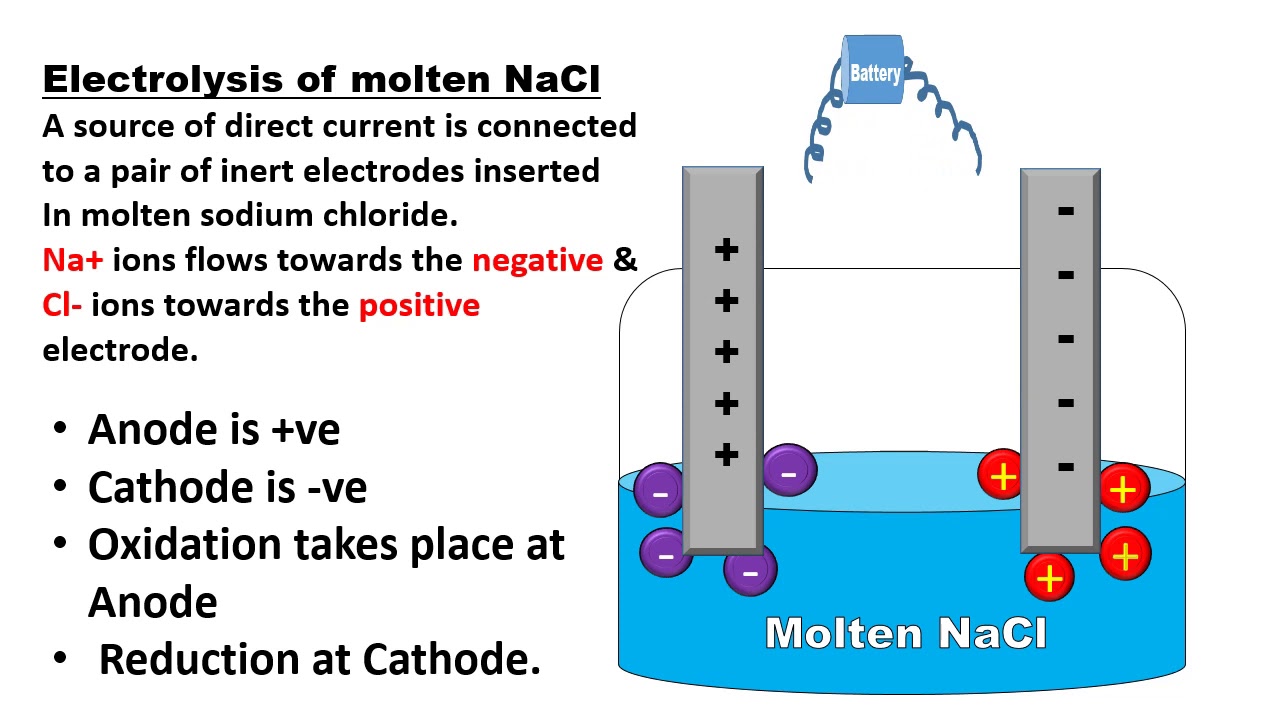
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas
No comments for "Anode and Cathode in Electrolysis"
Post a Comment